Attend
Patient Engagement Solutions & Innovations World Congress 2026 Europe
Transforming Care Through Technology, Empathy, and Collaboration.
London, United Kingdom
Thursday 26th - Friday 27th March 2026
We are delighted to welcome you to our upcoming PATIENT ENGAGEMENT SOLUTIONS & INNOVATIONS World Congress 2026 Europe, hosted by FACILITATE LIVE. This year’s congress focuses on “Transforming Care Through Technology, Empathy, and Collaboration.“
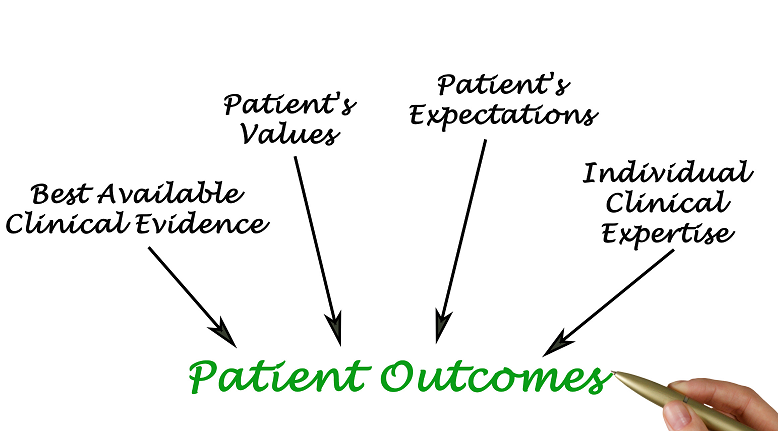
In an era defined by accelerated digital transformation, patient engagement has become a strategic imperative for the pharmaceutical and healthcare sectors. Beyond improving clinical outcomes, it is reshaping how organizations create value, differentiate therapies, and build trust in a data-driven, patient centric ecosystem.
The convergence of AI, real world evidence, digital therapeutics, and precision medicine is enabling more targeted interventions and personalized care pathways. Connected platforms spanning remote monitoring, predictive analytics, and patient support programs—are transforming engagement across the product lifecycle, from clinical development to post-market management. Patients are now active participants, empowered through access to information, digital tools, and real-time feedback loops that drive better adherence and informed decision-making.
For pharmaceutical leaders, this shift presents an opportunity to integrate patient perspectives into every stage of innovation. Digital trials and decentralized research models expand participation and enhance diversity, while AI-driven insights enable faster recruitment, improved trial design, and more representative data. Post-launch, digital engagement solutions sustain communication, reinforce adherence, and generate real-world data that strengthens value demonstration and market access. As healthcare systems evolve toward value-based and outcomes driven models, patient engagement sits at the intersection of innovation, access, and accountability. Success will depend on the industry’s ability to harness data interoperability, responsible AI, and ethical data governance to ensure transparency, privacy, and equitable participation.
However, challenges remain. Digital inequity, fragmented infrastructures, data silos, and clinician resistance continue to slow progress. Overcoming these barriers will require cross sector collaboration, regulatory alignment, and a shared commitment to patient-centered design. Investment in education, digital literacy, and ecosystem interoperability will be critical to sustaining engagement and trust. At this congress, our goal is to unite leaders across healthcare and life sciences to align on a shared vision for digital maturity and connected care. Together, we will explore actionable strategies to accelerate adoption, measure impact, and scale innovation that truly serves patients.
The next era of healthcare will be defined not just by technological advancement, but by how effectively we integrate empathy, evidence, and innovation to deliver meaningful, measurable outcomes. Patient engagement is no longer optional—it is the foundation of sustainable growth and improved global health.
We look forward to meeting you at the Congress!
GAIN LATEST INSIGHTS ON:
- Critical topics regarding patient centricity within the pharmaceutical and life sciences industry.
- Why it’s imperative for our industry to fully embrace the patient-centric movement?
- We will delve into several key areas that are vital for its successful implementation.
- We will be exploring what patient centricity truly means for our sector.
- Why it’s so crucial for the industry to actively participate in this shift?
- Patient-centric initiatives that have already demonstrated a significant impact.
- Successful strategies and best practices for managing patient centricity in healthcare.
- How we can develop and execute effective strategies to improve patient engagement and the benefits this engagement brings to the drug development and discovery process.
- Evolving challenges and key considerations in the ethical and compliant use of relevant data, and the potential value of providing access to this data to all stakeholders.
- How we can maintain mutually beneficial ongoing partnerships between patients and the industry.
- Overcoming the challenges in making clinical trials and drug development more patient-centered.
- Setting expectations through collaborations
- Understanding the role of researchers in bridging communication gaps.
- This congress also focuses on practical strategies for developing a powerful brand with the patient at its core.
WHO SHOULD ATTEND?
This Congress is beneficial to pharmaceutical, biotech companies, researchers, physicians, patient advocacy groups, regulatory agencies, technology and healthcare companies.
Network with Presidents, Heads/Chiefs, VPs, Directors, and Leaders in the area of:
- Patients and Caregivers
- Clinical Research Professionals
- Clinical Trial Coordinators
- Patient Advocacy and Engagement
- Pharmaceutical Marketing and Communication
- Medical Affairs Executives
- Regulatory Affairs Specialists
- Data and Real-World Evidence Analysts
Chief Information Officers - Chief Technology Officers
- Software Engineers and App Developers
- AI, Machine Learning, and Data Analytics
Professionals - Digital Health Specialists
- Product Development Leads
- Doctors, Physicians, and Surgeons
- Nurses and Nurse Practitioners
- Allied Health Professionals
- Hospital and Clinic Administrators
- Medical Directors and Clinical Managers
- Patient Experience Officers
- Care Coordinators
- Healthcare CEOs, COOs, and Innovation Officers
- Health Policy Makers and Regulators
- Public Health Officers
- Academics and Researchers
- NGO and Nonprofit Representatives
- Insurance and Payer Representatives
- Investors and Venture Capitalists
- Healthcare Consultants and Analysts
- Start-up Founders and Entrepreneurs
- Patient Advocacy Groups


Delegate Tickets
Group Discounts Are Also Available
Patients / Patient Group
Subject To Approval- Full Access - 2 Day Conference
- All Day Refreshments & Lunches
- Conference Documentations
- Networking Drinks Reception
Non Profit / Acedemic
Verification Required- Full Access - 2 Day Conference
- All Day Refreshments & Lunches
- Conference Documentations
- Networking Drinks Reception
Early Bird Discount -Vendor Registration Rate
Vendors / Service / Solution Providers / Consultants / CRO's- Full Access - 2 Day Conference
- All Day Refreshments & Lunches
- Conference Documentations
- Networking Drinks Reception
Standard Vendor Registration Rate
Vendors / Service / Solution Providers / Consultants / CRO's- Full Access - 2 Day Conference
- All Day Refreshments & Lunches
- Conference Documentations
- Networking Drinks Reception
Standard Registration Rate
Pharma / Biotech- Full Access - 2 Day Conference
- All Day Refreshments & Lunches
- Conference Documentations
- Networking Drinks Reception